
Contact number
Sino-Biocan(Shanghai) Biotech Ltd
2nd Floor, Building 15, No.6055, Jinhai Road, Fengxian District, Shanghai
Contact number
021-60890755
Business Department
Name:罗嘉 Luo Jia
Position:全国业务总监
Telephone:86 13901096635
E-mail:luojia@sinobiocan.com
市场与企业策划部
Name:
Position:联系我们
Telephone:
E-mail:info@sinobiocan.com
Date:2022-03-15Edit:Sino-Biocan
Sino-Biocan(Beijing) Biotech Ltd(hereinafter referred to as “Sino-Biocan”) has recently completed a Series A round of funding of nearly RMB 100 million,led by Grand Flight Investment and continued by its long-time shareholder, Sinovation Ventures.This round of financing will be used to expand the new R&D and design institute (including R&D centre, design centre, testing centre, data centre, customer service centre and after-sales service centre) and expand the R&D pipeline, further build and improve the capacity management of fully automated equipment, GMP-grade disposables, GMP and clinical test-grade liquid production at Shanghai production base. We are also improving technical service for existing products and cell preparation tools. We aim to develop a multi-marketing approach to meet the demand of recent rapid growth in original product development for multiple pipelines of differentiated cellular and genetic drugs, in line with accelerated drug declaration and orderly capacity expansion and meet the needs of a wide range of GMP-grade cell preparation markets.
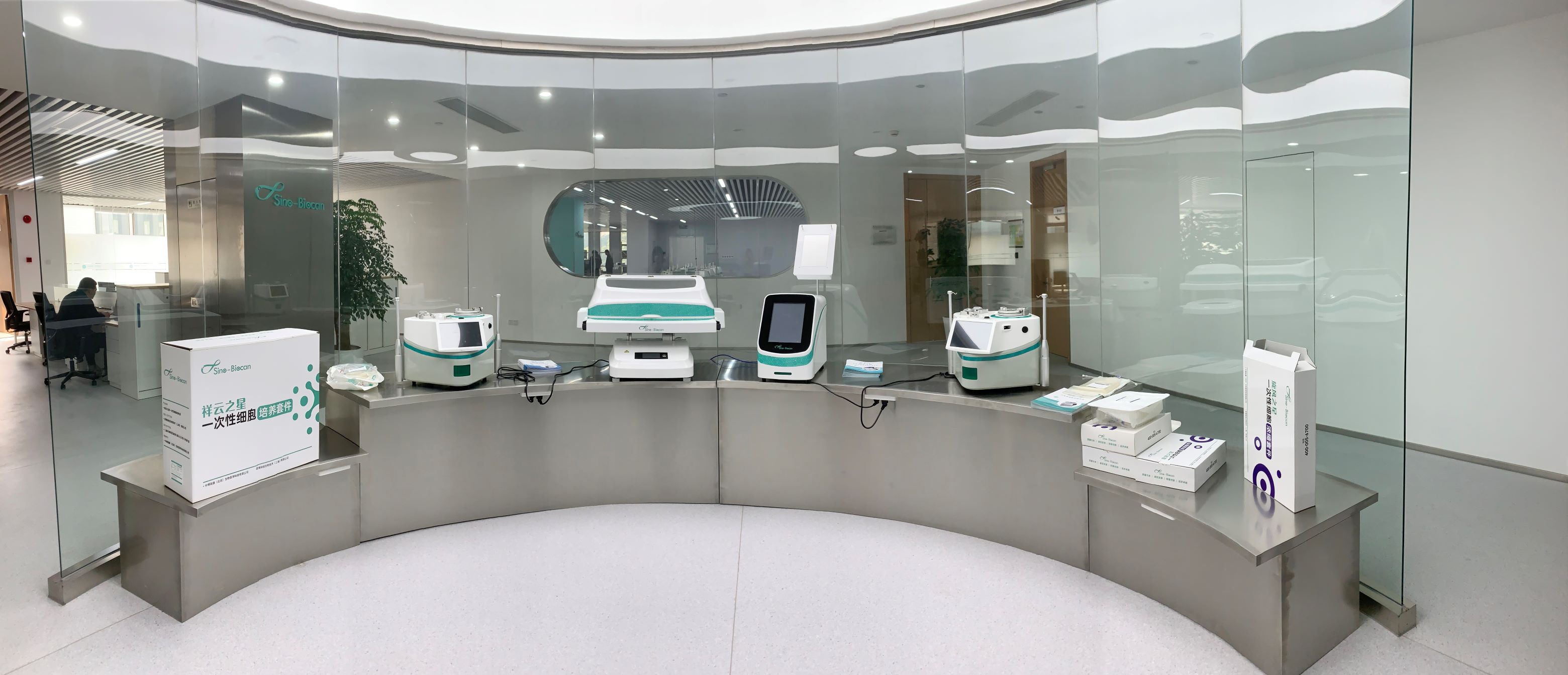
Sino-Biocan has developed a GMP-grade product family of fully closed, modularized, continuous cell preparation tools, as well as consumables and liquid solution, covering processes such as automated cell separation, culture, cell concentration, washing and formulation filling, cryopreservation and recovery, widely applied in cell and gene drug development, CDMO, medical and pharmaceutical stem cell preparation and application, cord blood bank management and cell storage, etc. We can also provide modular splicing of different GMP processes according to specific customer requirements for different pipelines, to realize the rapid, efficient and differentiated customization of series products of cell preparation tools.

In 2021, Sino-Biocan has completed the construction of its self-owned production base with a total area of 7600㎡in Shanghai Lingang Nanqiao Science and Technology City, which includes GMP-grade C+A consumable clean workshop, pharmaceutical grade liquid clean workshop, NMPA medical device production workshop, C+A cell & gene therapy process research and validation lab, equipment R&D lab, high clean level fully functional QC center, high level low-temperature and room-temperature warehouse, reaching production scale of 200,000 sets of consumables, 600,000 bottles of liquid reagents and 3,000 sets of equipment per year for cell & gene therapy preparation. In 2024, Sino-Biocan will expand the second-phase production base of more than 14,000㎡, intending to make it a leading production base for cell & gene therapy intelligent tools both in China and worldwide.

The founder of Sino-Biocan, Shirley(Wei Dongbing), spent 13 years in one of the Fortune Global 500 medical device company before taking over as the general manager of China for the world's largest immunization company, Miltenyi Biotec, where she set up R&D, product and marketing departments and served thousands of customers.With more than 20 years of experience in international companies in cell and gene field, Shirley founded Sino-Biocan(Beijing) Biotech. She contributes herself to building up Sino-Biocan into a leading international company with in-depth insight into the needs of cell preparation tools, excellent commercialization capabilities, and excellent experience in R&D and production management.